Garnet Solid Electrolytes
Bi-functional Na Electrodes
Tetrahedrite Thermoelectrics
1) Structure and Dynamics of Lithium Garnet Oxides
Liquid electrolytes in the state-of-the-art Li-ion batteries contain volatile and flammable organic solvents, which has raised safety concerns over the wide adoption of these batteries in large-scale automotive, aeronautic, stationary applications. Solid electrolytes with high ionic conductivity and good chemical/electrochemical stability could potentially revolutionize the electrode and cell designs and provide superior safety in batteries.
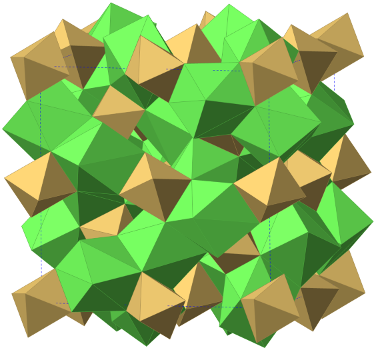
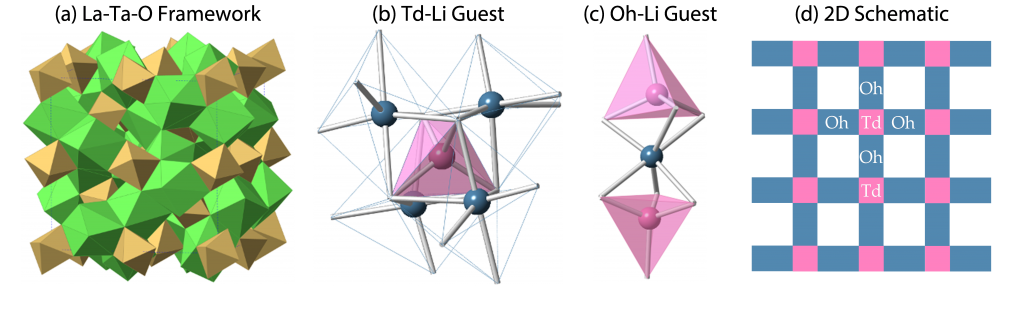
We are investigating a prototypic series of ionic materials, lithium garnet oxides Li7-xLa3Zr2-xTaxO12 (x=0-2). The framework of the materials is composed of LaO8 dodecahedra and TaO6 octahedra (Figure a). There are two types of cages, tetrahedral (Td) and octahedral (Oh), to host lithium ions (Figure bc). Each Td cage is surrounded by four Oh cages and each Oh cage is surrounded by two Td cages (Figure d). Both the Td and Oh cages are only partially occupied. The scientific goal of our research is to understand structure and dynamics of lithium disorder and their effect on lithium ionic conduction. This work is supported by National Science Foundation.
2) “Bi-functional” Electrode Materials for Na-ion Batteries
While Li-ion batteries have dominated the portable electronics market and started their penetration into the transportation and stationary markets, there is growing concern over the lithium abundance and geographical constraints of lithium minerals. Sodium element is more than 1000 times more abundant than lithium in earth’s crust and sea and sodium resources are considered practically unlimited.
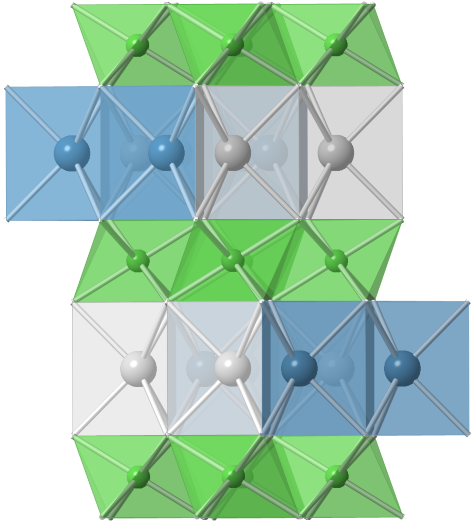
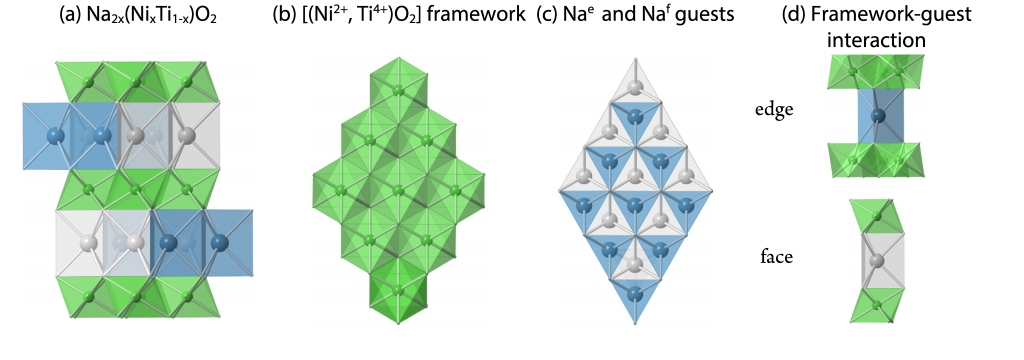
We are studying a class of mixed ionic-electronic conducting materials, Na2x(NixTi1-x)O2, as electrodes for Na-ion batteries. The structure consists of a layered framework of NiO6 and TiO6 octahedra and Na guests in two different cages, i.e. edge and face-shared NaO6 prisms (Figure). Since their compositions include both the high redox-potential transition metal element Ni, and the low redox-potential transition metal element Ti, they can be utilized as either a negative or positive electrode, i.e. “bi-functional”. We have demonstrated the “bi-functionality” of these materials and are currently investigating the relationship between their structures and electrochemical properties. This work is supported by National Science Foundation.
3) Structure and Dynamics of Thermoelectric Materials
Currently around two-thirds of energy produced in US is rejected mainly in the form of waste heat. Such unused heat can be recovered by thermoelectric processes that directly convert thermal energy into electricity. Robust and cost-effective thermoelectric devices could have significant impact on the energy production and utilization of the society.
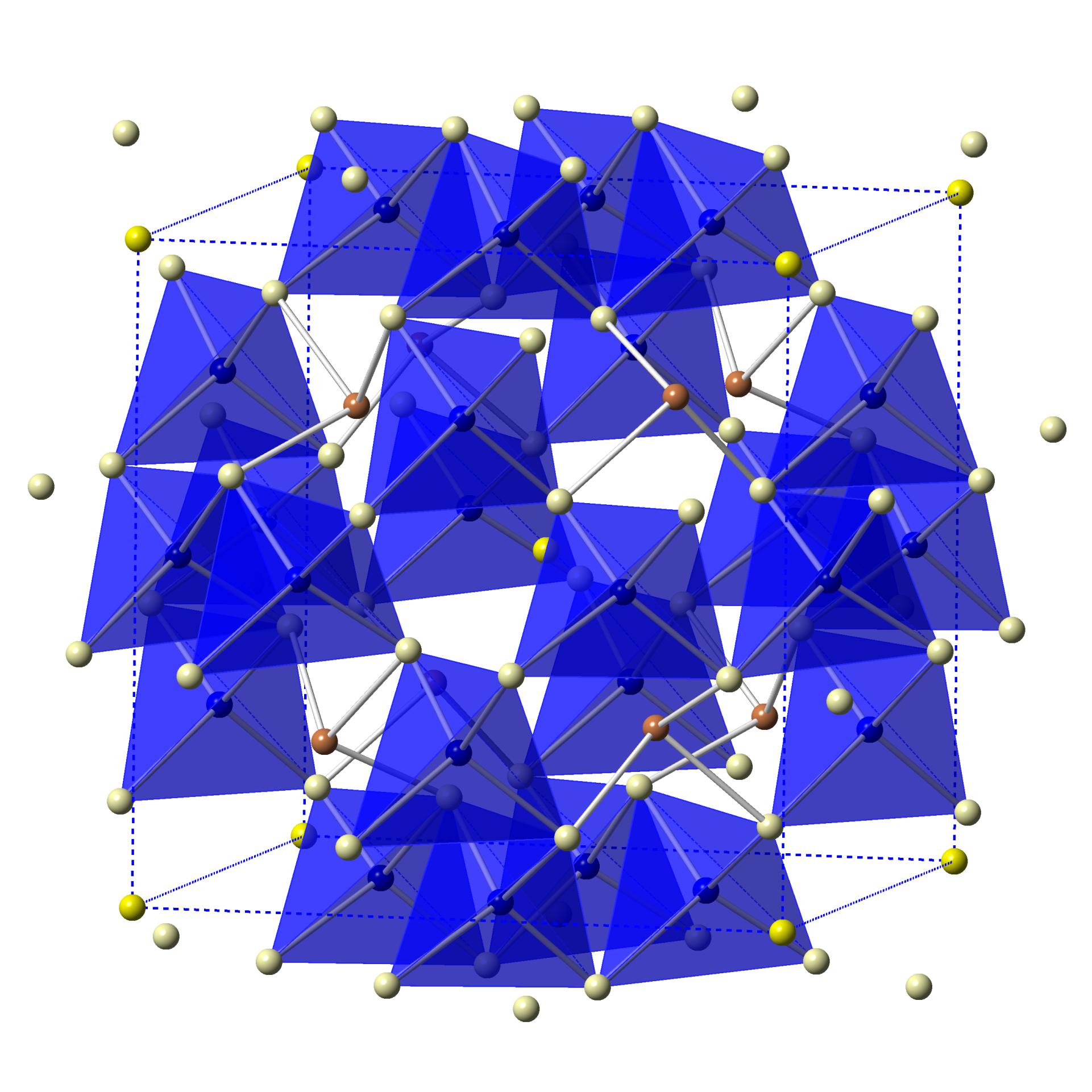
Tetrahedrites are a class of materials based on Cu12Sb4S13 containing earth-abundant and environmentally friendly elements. The structure consists of a 3D framework of CuS4 tetrahedra and SbS3 polyhedra (Figure a). Another type of Cu atoms (Cu12e) behave as rattling guests inside a cage formed by three S and two Sb atoms (Figure b). The interaction between Cu12e and Sb atoms is mitigated by the Sb lone pairs (Figure c). We are currently investigating the relationship between atomic and electronic structure and thermoelectric properties of undoped and doped Cu12Sb4S13 tetrahedrites. This work is in collaboration with my colleague Donald Morelli here at MSU and is supported by National Science Foundation.

